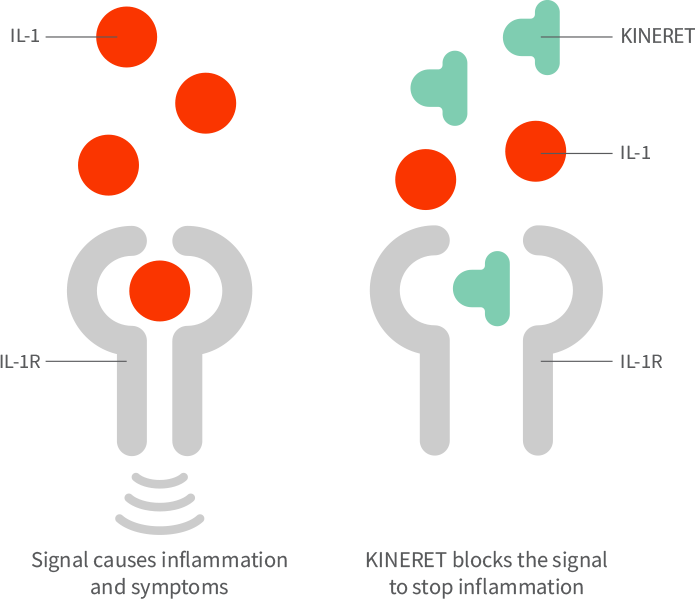
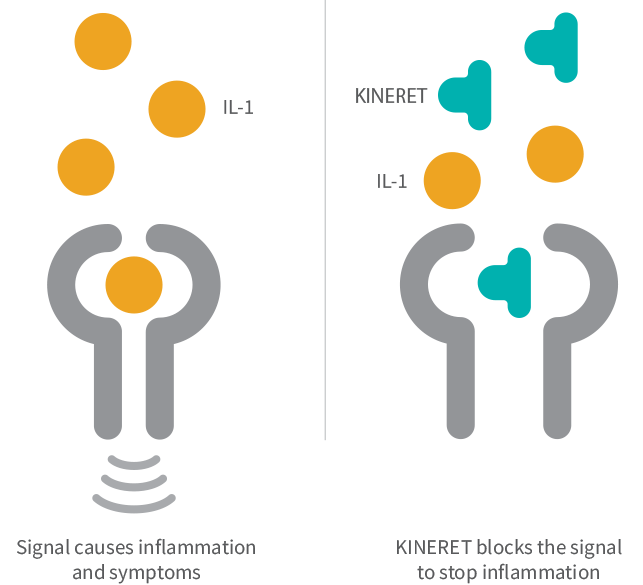
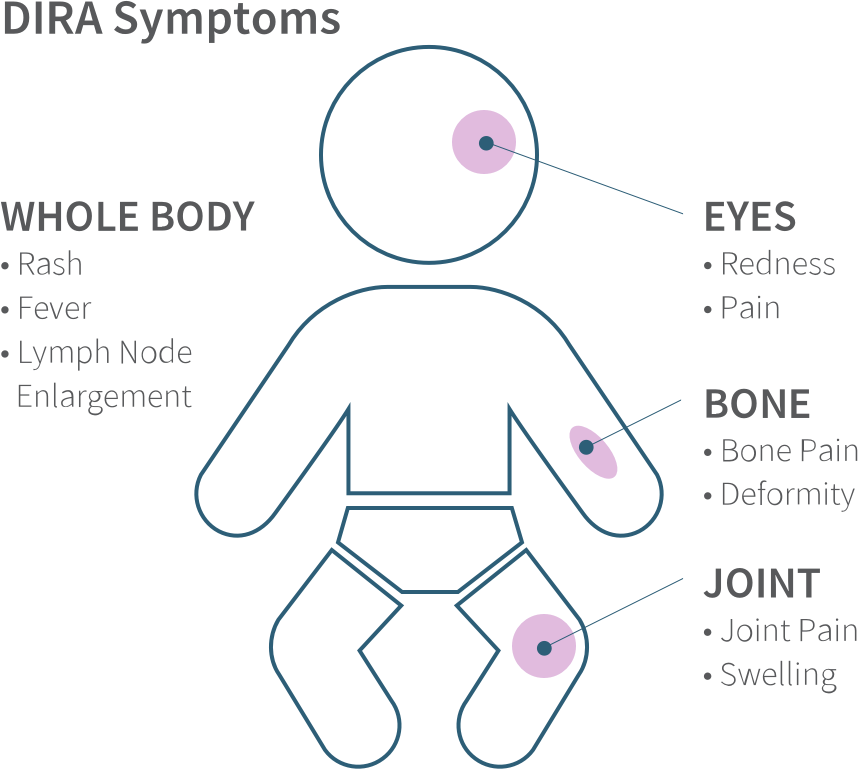
Do not take KINERET if you are allergic to:
- Proteins made from bacteria called E. coli. Ask your healthcare provider if you are not sure
- Anakinra or any of the ingredients in KINERET. See the end of the patient leaflet for a complete list of ingredients in KINERET
Before starting KINERET, tell your healthcare provider if you:
- Have an infection, a history of infections that keep coming back, or other problems that can increase your risk of infections
- Are scheduled to receive any vaccines. People using KINERET should not receive live vaccines
- Have kidney problems
- Are pregnant or plan to become pregnant. It is not known if KINERET will harm your unborn baby
- Are breastfeeding or plan to breastfeed. It is not known if KINERET passes into your breast milk. You and your healthcare provider should decide if you will use KINERET or breastfeed
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
KINERET and other medicines may affect each other and cause serious side effects.
KINERET may cause serious side effects, including:
Serious infections. KINERET may lower your ability to fight infections. Call your healthcare provider right away if you get an infection, have any sign of an infection including a fever or chills, or have any open sores on your body. Due to the risk of infection, you should not receive live vaccines while you use KINERET.
Serious allergic reactions. Stop using KINERET, call your healthcare provider, and get emergency help right away if you have any of these symptoms of a serious allergic reaction: swelling of your face, lips, mouth, or tongue; trouble breathing; wheezing; severe itching; skin rash, redness, or swelling outside of the injection site area; dizziness or fainting; fast heartbeat or pounding in your chest (tachycardia); or sweating. People with DIRA may have an increased risk of allergic reactions, especially in the first several weeks.
Decreased ability of your body to fight infections (immunosuppression). It is not known if treatment with medicines that cause immunosuppression, like KINERET, affect your risk of getting cancer.
Amyloidosis. KINERET may cause a buildup of abnormal proteins called amyloids around the injection site (a hard lump under the skin). Avoid using the same site to inject KINERET by rotating to alternating areas of the body. Your doctor may monitor you for amyloidosis.
Low white blood cell count (neutropenia). KINERET may cause you to have a lower number of certain white blood cells (neutrophils). Neutrophils are important in fighting infections. Your doctor should do blood tests to check your white blood cell level before starting and during the first year of treatment with KINERET.
The most common side effects of KINERET include:
- Injection site skin reactions. The symptoms of injection site skin reactions may include: redness, swelling, bruising, itching, and stinging
- RA gets worse with treatment, if you already have RA
- Headache
- Nausea and vomiting
- Diarrhea
- Joint pain
- Fever
- Feeling like you have the flu
- Sore throat or runny nose
- Sinus infection
- Pain in your stomach area
Tell your healthcare provider if you have any side effect that bothers you or does not go away.
These are not all of the possible side effects of KINERET. For more information ask your healthcare provider or pharmacist. You can also see the Full Prescribing Information for KINERET including Patient Information and Instructions for Use at https://www.kineretrx.com/pdf/Full-Prescribing-Information-English.pdf
To report suspected side effects, contact Sobi North America at 1-866-773-5274 or FDA at 1-800-FDA-1088.